Abstract
Glucocorticoids (GCs) are a cornerstone of front-line T-cell acute lymphoblastic leukemia (T-ALL) treatment. While mutations in NR3C1, which encodes the GC receptor (GR), and other genes involved in GC signaling occur at relapse, other mechanisms of adaptive GC resistance are largely unknown. In an unbiased in vivo screen to discover GC resistance mechanisms, we transplanted and treated 10 primary mouse T-ALLs initiated by retroviral insertional mutagenesis with the GC dexamethasone (DEX) (Wandler et al., 2020). GR protein expression was reduced in ~40% of resistant clones overall, including those with Nr3c1 mutations. The remaining 60% of primary relapsed/resistant leukemias are a valuable resource for discovering additional mechanisms of adaptive GC resistance.
Using shotgun cloning, we identified new and unique retroviral integrations near Jdp2 with a corresponding increase in expression in different relapsed clones from independent recipient mice transplanted with T-ALL 8633. Jdp2 integrations were not present in any of the other 9 leukemias. Jdp2 was identified as a T-ALL oncogene in previous insertional mutagenesis screens and shown to induce de novo GC resistance in zebrafish thymocytes (Mansour et al., 2018). T-ALL 8633 was the only leukemia in our panel with a Kdm6a mutation, and we hypothesized that Jdp2 and Kdm6a might interact to modulate GC sensitivity.
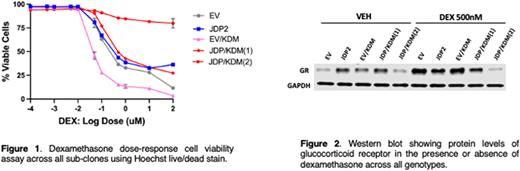
To explore a possible functional interaction between Jdp2 and Kdm6a in GC resistance, we over-expressed JDP2 using lentiviral transduction and inactivated KDM6A using CRISPR/Cas-9 in CCRF-CEM human T-ALL cells. We refer to these cells as EV (empty vector controls), JDP (JDP2-expressing), EV/KDM (KDM6A mutant) or JDP/KDM (JDP2-expressing; KDM6A mutant). The experiment was reproduced in a second independent CCRF-CEM subclone with similar findings. JDP2 over-expression alone conferred minimal DEX resistance in CCRF-CEM cells (Figure 1). By contrast, KDM6A inactivation unexpectedly enhanced GC sensitivity. Interestingly, JDP2 over-expression reversed this sensitivity in one doubly mutant S1 subclone (JDP/KDM1) and induced overt DEX resistance in another (JDP/KDM2). DEX exposure induced cleaved caspase 3 (CC3) expression in CCRF-CEM subclones that correlated with drug sensitivity, with EV/KDM cells showing the highest levels of CC3 and cells expressing JDP2 alone the lowest. Together, these data suggest a model whereby loss-of-function mutations in KDM6A enhance the sensitivity of T-ALL cells to DEX-induced apoptosis, which then results in selective pressure favoring outgrowth of resistant clones with elevated JDP2 expression.
We next investigated basal and DEX-induced NR3C1 and GR expression levels. Upon exposure to DEX, EV/KDM cells up-regulated NR3C1 mRNA and GR protein levels to a greater extent than EV controls, whereas JDP cells were less responsive (Figure 2). Furthermore, "double-mutant” JDP/KDM subclones generally behaved similarly to the JDP2 over-expressing cells, supporting the idea that JDP2 can overcome the DEX sensitization conferred by KDM6A inactivation. To address the potential clinical relevance of these data, we analyzed genomic data from 103 pediatric ALL patients (Li et al., 2020). Of 9 patients with T-ALL and available paired transcriptome data from diagnosis and relapse, two harbored KDM6A mutations. One of these leukemias acquired a somatic NR3C1 mutation at relapse and the other uniquely showed a ~10-fold increase in JDP2 mRNA expression. This relapsed T-ALL also had reduced NR3C1 expression as compared to the paired diagnostic sample.
Altogether, these data implicate elevated JDP2 expression as a driver of intrinsic and acquired GC resistance in T-ALL by blocking the expected increase in GR expression in lymphoblasts upon GC treatment. Our findings are consistent with other recent studies showing that genetic and epigenetic mechanisms that converge on GR signaling play a central role in ALL relapse (Li et al., 2021; Autry et al., 2020). We also unexpectedly found that KDM6A inactivation sensitizes T-ALL cells to GC treatment and hypothesize that this results in strong selective pressure for the outgrowth of GC-resistant clones due to JDP2 over-expression and other mechanisms. The FDA-approved BCL-2 inhibitor venetoclax and H3K27me3 inhibition are rational therapeutic approaches for potentially overcoming adaptive GC resistance in relapsed patients with KDM6A-mutant T-ALL.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal